High-resolution ESI-FTICR-MS was applied for analysis of the degradation products generated during storage of PMMA and PS polymers synthesized in a RAFT polymerization in THF. In E2 elimination shows a second order rate law and occurs in a single concerted step proton abstraction at C α occurring at the same time as C β-X bond cleavage.
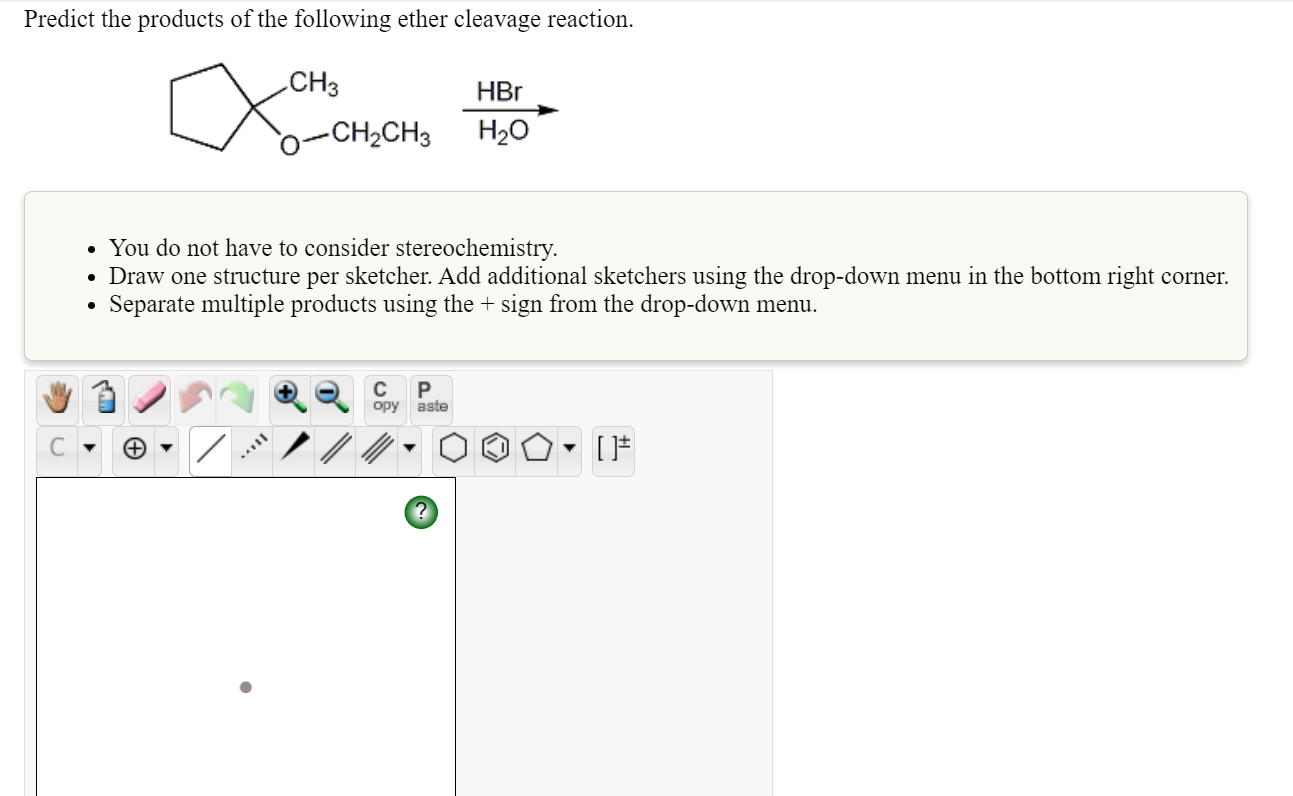
Solved Predict The Products Of The Following Ether Cleavage Chegg Com
TUTORIAL 40 INTRODUCTION TO ORGANIC CHEMISTRY CH3 CH3 H H3C C CH2 H3C C CH2CH3 CH3 H3C C CH2CH3 H 8.
. In E1 elimination goes via a first order rate law in two steps C β-X. The greatly increased boiling point is due to the fact that butanol contains a hydroxyl group which is capable of hydrogen bonding. Alkyl halides undergo elimination via two common mechanisms known as E2 and E1 which show some similarities to S N 2 and S N 1 respectively.
Strong nucleophiles will also open the strained ether as shown by reaction 3b. Since you have posted more than one question we can do only first question for you. Most likely to be useful only to students in courses for chemistry.
These polymers carried cumyldithiobenzoate end groups which were essential for subsequent. In Naproxen is the lone pair of the oxygen atom delocalize. Molecules of diethyl ether C4H10 O are held together by dipole-dipole interactions which arise due to the polarized C-O bonds.
The rules are then applied to the products from the. In view of the catalysts reported to date the mechanism of the RWGS reaction can be assigned to either the decomposition of HOCO intermediates or to direct CO bond cleavage to produce CO 75. Draw the major product of the following reaction with mechanism.
In Naproxen is the lone pair of the oxygen. Example 5 is an interesting case of intramolecular rearrangement to an ortho-ester. Free oxirane groups couple via stable ether bonds with hydroxyl-containing molecules such as sugars via alkylamine linkages with ligands containing amino groups and via thioether linkages with ligands containing thiol groups.
Arrange the following carbocations in order of increasing stability. Still the attractive forces in. The problems have been color-coded to indicate whether they are.
A nanoporous Ni-Cu mixed surface greatly enhances the electrochemical conversion of HMF to FDCA involving stepwise oxidations of alcohol to aldehyde and carboxylic acid on. Synthesis reaction worksheet organic chemistry. Predict the major product for the following reaction.
TOSOH Chromatographic Media Catalog TOSOH TOYOPEARL. 52 Comparison of epithilones B 10 and D 11 which differ only by the presence of. Give one example for each cleavage.
The following problems are meant to be useful study tools for students involved in most undergraduate organic chemistry courses. A handy rule to remember for this purpose is the following. David Rawn in Organic Chemistry Second Edition 2018 The S N 2 Mechanism.
Academiaedu is a platform for academics to share research papers. Following their synthetic work toward the natural products the Danishefksy laboratory began to apply a medicinal chemistry approach seeking to establish a structureactivity relationship SAR with the hope of ultimately arriving at an optimized clinical candidate. Cleavage reactions of β-lactones may take place either by acid-catalyzed acyl exchange as in 4a or by alkyl-O rupture by nucleophiles as in 4b.
But no according to my professor ether would be the best one because it. The results could be used to predict the long-term behavior during a nuclear waste storage time. Im other words can the oxygen A.
In a SN2 reaction ammonia is the nucleophile and it is asked which solvent will make the reaction faster. The conjugate base. The algorithm applies iteratively the generalized reaction rules to a set of starting compounds and a set of enzyme cofactors.
Use amino acid characteristics to predict hydrophobicity. I would say ethanol because it would stabitize the transition state by solvation right. Compare its boiling point of 35 Cwith that of Its isomer butanol 117 C.
Finally the β-lactam cleavage of penicillin G reaction 6. Because the reaction occurs in one step it is concertedThe substrate and the nucleophile are both present in the transition state. If heat is observed as products were formed means that the reaction is ___.
The S N 2 mechanism is a one-step process in which a nucleophile attacks the substrate and a leaving group L departs simultaneously. Each starting compound is evaluated to determine if it contains the functional group that will be transformed by the reaction rules generating all possible products. Most likely to be useful to students in year long rather than survey courses 3.
B Classify each of the following as electrophile nucleophile or free radical. If you want question_answer. A Define the terms electrophile and nucleophile.
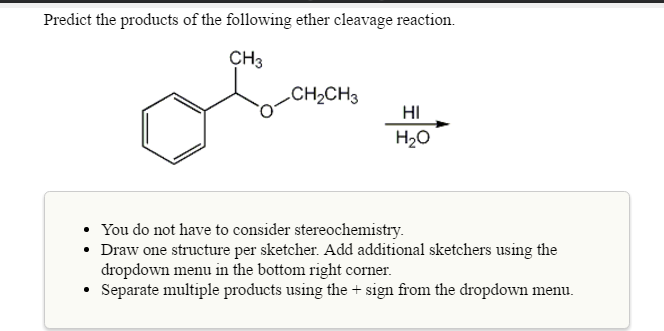
Solved Predict The Products Of The Following Ether Cleavage Chegg Com

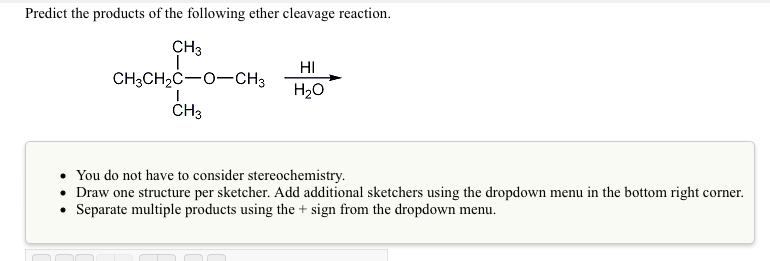
Solved Predict The Products Of The Following Ether Cleavage Chegg Com
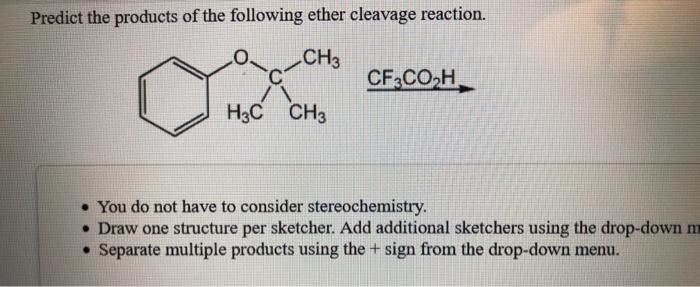
Solved Predict The Products Of The Following Ether Cleavage Chegg Com
0 Comments